Thermochemical cycle for water splitting promises an efficient way to convert thermal energy to chemical energy stored in hydrogen. Water is split into stoichiometric amounts of hydrogen and oxygen in different steps of the thermochemical cycle with heat as the sole energy source. There are generally two categories of thermochemical cycles for water splitting: 1) high-temperature two-step cycles and 2) low-temperature multistep cycles. An important tradeoff between system complexity and operating temperature exists for the two types of thermochemical cycles: two-step thermochemical cycles mostly involve gas-solid redox reactions of simple metal/metal oxides while operate at elevated temperatures (>1500°C). In contrast, multistep cycles offer operating temperatures well below 1000°C, however, they usually employ highly corrosive and toxic chemicals, e.g. the piloted sulfur-iodine cycle involves at least one of SO2, H2SO4, I2 and HI in each step.
The overarching objective of the thermal water splitting project is to develop a non-toxic, non-corrosive materials-based thermochemical cycle for water splitting with the highest operating temperature below 1000°C. Heat sources at temperatures above 1500°C, e.g. large-scale solar concentrators, are scarce. Due to the corrosiveness of high temperature steam, steam engines usually operate at below 600°C. Therefore, an efficient way to utilize heat sources at temperatures 600-1000°C is highly desirable.
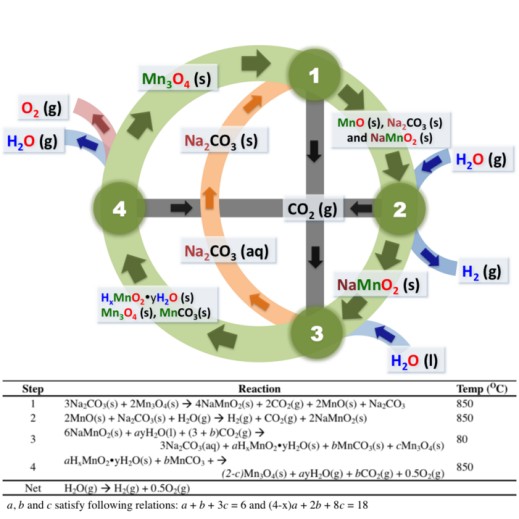
We recently demonstrated that a manganese oxide-based thermochemical cycle for water splitting can be closed at 850°C, with no toxic/corrosive chemicals involved. (http://www.pnas.org/content/109/24/9260.full.pdf). The mechanism of the cycle is schematically shown in the figure below.
Selected Recent Publication:
Xu, B; Bhawe, Y; Davis, ME; (2012) Proc. Natl. Acad. Sci. USA 109 9260-9264.
